24 hours a day, while the body is constantly working, cells are metabolizing and generating energy as they carry out their tasks. This natural process results in free radicals, the same way driving to work in your car creates exhaust fumes.
In our day to day activities, we are exposed to harmful free radicals which are present in our environment around us. Free radicals are chemically active atoms or molecular fragments that have a charge due to an excess or deficient number of electrons. Examples of free radicals are the superoxide anion, hydroxyl radical, transition metals such as iron and copper, nitric acid, and ozone.
Free radicals are unstable molecules within the body that attack and destroy the biochemical processes within cells and compromise the immune system which makes the body vulnerable to disease. These free radicals causes oxidative stress to our bodies. Oxidative Stress (1) or Oxidation means rusting process. Free radicals are also known as Oxidants and to neutralize them you need to take AntiOxidants.
Oxidative stress means an unbalance between pro-oxidants and antioxidant mechanisms. This results in excessive oxidative metabolism. This stress can be due to several environmental factors such as exposure to the sun, pesticides and other kinds of environmental pollution. In addition, their levels are increased by mental and physical stress, the consumption of alcoholic beverages, unhealthy foods, and cigarette smoke (see below).
Many factors can induce free radical formation in the environment. They cause clothes to fade, food to spoil, metal to rust, pipes to leak, the deterioration of plastics, the fading and peeling of paint and the degradation of works of art. Examples of free radicals are the superoxide anion, hydroxyl radical, transition metals such as iron and copper, nitric acid, and ozone.
In the human body free radicals can be useful because they help important reactions to take place. Free radicals arise normally during metabolism and sometimes the immune system’s white blood cells purposefully create them to neutralize bacteria and viruses. Free radicals are a natural byproduct of the body when it turns food into energy and, normally, the body makes its own antioxidant enzymes to deal with them. Catalase, glutathione peroxidase and superoxide dismutase are three such enzymes and they require micronutrient co-factors such as copper, iron, manganese, selenium and zinc for their activity.
Excess free radicals are a problem because they attack the body itself, damaging key cellular molecules such as DNA. Cells with damaged DNA are more prone to developing cancer and free radical damage accumulates with age.
Possible results of Free Radical damage
- Arthritis
- Adrenal fatigue
- Alzheimer’s disease
- Attention deficit hyperactivity disorder (ADHD)
- Atherosclerosis
- Autism
- Bipolar disorder
- Cancer
- Cataract formation
- Chronic fatigue syndrome
- Crohn’s disease
- Depression
- Diabetes
- Fibromyalgia
- Heart disease
- Huntington’s disease
- Hypertension
- Hyperthyroidism
- Inflammation and pain
- Bladder and kidney stones
- Macular degeneration
- Methylenetetrahydrofolate reductase (MTHFR) gene mutations
- Multiple sclerosis
- Parkinson’s disease
- Premature ageing
- Rheumatoid arthritis
- Stroke
- Tinnitus
Normally the body is able to deal with this natural process when a variety of antioxidants are abundant in our daily diet. However due to today’s lifestyles most of us are producing far too many free radicals for the normal body processes to cope with.
Where Do The Free Radicals Come From?
A series of chemical reactions that changes molecules to different molecules is called a metabolic pathway. Metabolism of food is the numerous pathways involved in breaking food down for building blocks (spare parts) and for extracting and storing the energy from food. Metabolism of the body includes the building of molecules from spare parts needed to keep the chemical reactions of the body going and to maintain its structure.
The major source of FRs and oxidants is the mitochondrial generation of ATP energy using oxygen. Another words, when cells create energy , they also produce unstable oxygen molecules. It means y our normal bodily functions (breathing, metabolism, and physical activity) naturally generate free radicals.
FRs also is by-product of many of detoxification reactions in our body. For example, cytochrome P450 enzymes are source of oxidants. These enzymes help cells, especially in the lungs and liver, detoxify a broad range of potentially toxic food, drug and environmental pollutant molecules.
Finally, when white blood cells attack germs and viruses this may create serious FR problems, especially in those suffering a chronic immune-activation condition, such as AIDS, chronic candidiasis, protozoal infections, chronic fatigue syndrome, etc. It means y our immune system also generates free radicals to help neutralize viruses and bacteria.
There are four primary sources of free radicals:
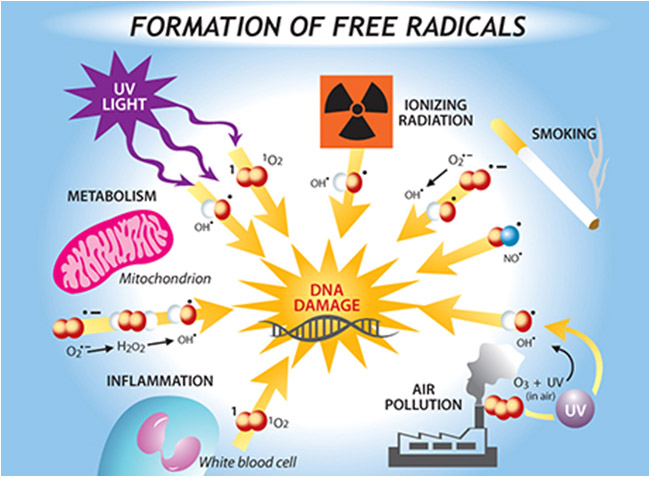
- The Environment (exogenous) free radicals come from outside the body from environmental factors, like the factors listed below. Air pollution, cigarette smoke, smog, soot, automobile exhaust, toxic waste, pesticides, herbicides, ultraviolet light, background radiation, drugs, and even certain foods can all generate free radicals in the body.
- Internal Production. (endogenous) Our bodies constantly produce free radicals as a byproduct of normal metabolism. And yes, since exercise increases metabolic rates, it increases the production of free radicals. Free radicals are created inside cells during metabolism of food molecules or in the other words the oxidants that develop from processes within our bodies form as a result of normal aerobic respiration, metabolism, and inflammation (see above).
- Stress Factors. Aging, trauma, medications, disease, infection, and "stress" itself all accelerate the body's production of free radicals -- by a factor of eight, or more.
- Chain Reactions. When a free radical steals an electron to balance itself, it creates a new free radical in the molecule from which it stole the electron. In many cases, the new free radical will seek to balance itself by stealing its own electron -- and on and on. And remember, even one free radical is capable of destroying an entire cell, or a strand of DNA. Imagine what a chain reaction of millions of free radicals can do!
Causes of excess of free radicals in the body
- Alcohol
- Breathing
- Burnt food
- Charcoal cooking
- Excessive exercise (normal exercise does not have this effect)
- Excessive medications
- Food additives
- Fried food
- Fungicides, herbicides and pesticides
- Infections
- Irradiated food
- Overheated food
- Ozone
- Polluted environments
- Radon
- Radiation
- Smoking tobacco
- Stress
- Sunbathing
- Ultraviolet lights
- The process of metabolism and cell respiration
You can see from this list that many people will be producing far too many free radicals for the normal body processes to cope with, so they need to be sure that they take in enough foods with antioxidant abilities to deactivate these excess free radicals.
Let’s look at Chemical Bonding
To understand the way that free radicals and antioxidants interact, you must first understand a bit about cells and molecules. So here's a (very) brief refresher course in Physiology/Chemistry 101: The human body is composed of many different types of cells that, in turn, are composed of many different types of molecules. The following is an explanation of how free radicals are formed.
As you probably remember from your old high school days, atoms consist of a nucleus, neutrons, protons and electrons. The number of protons (positively charged particles) in the atom’s nucleus determines the number of electrons (negatively charged particles) surrounding the atom. 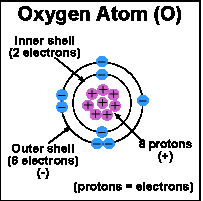
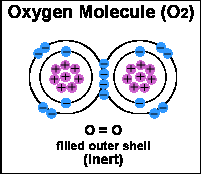
Electrons are involved in chemical reactions and are the substance that bonds atoms together to form molecules. Electrons surround, or "orbit" an atom in one or more shells. The innermost shell is full when it has two electrons. When the first shell is full, electrons begin to fill the second shell. When the second shell has eight electrons, it is full, and so on.
The most important structural feature of an atom for determining its chemical behavior is the number of electrons in its outer shell. A substance that has a full outer shell tends not to enter in chemical reactions (an inert substance). Because atoms seek to reach a state of maximum stability, an atom will try to fill its outer shell by:
- Gaining or losing electrons to either fill or empty its outer shell
- Sharing its electrons by bonding together with other atoms in order to complete its outer shell
Ions are electrically charged particles formed when atoms lose or gain electrons. They have the same electronic structures as noble gases. Metal atoms form positive ions, while nonmetal atoms form negative ions.
Atoms often complete their outer shells by sharing electrons with other atoms. By sharing electrons, the atoms are bound together and satisfy the conditions of maximum stability for the molecule. When it is difficult to take an electron from an atom, that molecule is known as being stable.

Noble gases argon, helium, krypton, neon, radon and xenon are the chemical elements in group 18 of the periodic table. They are the most stable due to having the maximum number of electrons their outer shell can hold. Therefore, they rarely react with other elements since they are already stable.
How Free Radicals Are Formed
An atom can lose an electron from its shell, leaving the atom with an unpaired electron (a free electron).
The new free electron pairs up with an electron of another atom, pushing out one of the electrons in the process and again creating a new free electron.
The displacing and replacing of electrons continues through hundreds and even thousands of atoms until finally two atoms with a free electron (spinning in opposite directions) pair up forming a bond between the two atoms.
Normally, bonds do not split in a way that leaves a molecule with an unpaired electron. But when weak bonds split, free radicals are formed. So, as we mentioned earlier the atoms that have a singular free electron are called free radicals. In the other words, these atoms have missing one or more electrons— they have at least one unpaired electron. This missing election is largely responsible for the process of biological oxidation . These "partial molecules" aggressively look to replace their missing parts by attacking other molecules.
These reactions are commonly referred to as "oxidation" reactions. A biogerontologist named Denham Harman first discovered the concept of free radicals in 1954, while researching an explanation for aging.
Free radicals are very unstable and react quickly with other compounds, trying to capture the needed electron to gain stability. They will attack the nearest stable molecule and steal its electron. When the attacked molecule loses its electron, it becomes a free radical itself, beginning a chain reaction, as it too will then attack and steal an electron from a nearby molecule.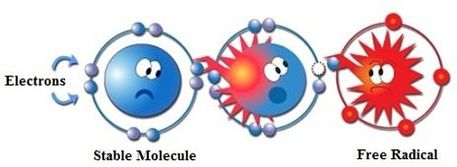
Once the process is started, it can cascade, like a domino effect, finally resulting in the disruption of a living cell. Thousands of free radical or so called chain reactions can occur within a few seconds of the primary reaction.
There Are Six Main Types of Free Radicals
Free radicals can be broken down into six types. The first four types come from oxygen atoms and are called Reactive Oxygen Species (ROS), but the fifth type derives from nitrogen:
- Superoxide ion (O):An oxygen molecule with an extra electron that can damage mitochondria, DNA and other molecules. This radical tries to steal its much-needed electron from the mitochondria of the cell. When mitochondria are destroyed, the cell loses its ability to convert food to energy. It dies.
- Hydroxyl radical (OH): A highly reactive molecule formed by the reduction of an oxygen molecule, capable of damaging almost any organic molecule in its vicinity, including carbohydrates, lipids, proteins, and DNA. This free radical attacks enzymes, proteins, and the unsaturated fats in cell membranes. OH cannot be eliminated by an enzymatic reaction.
- Singlet oxygen:Not technically a free radical, this metabolite can nevertheless wreak havoc on the body. It's listed here because it functions like a free radical and because it is controlled by antioxidants. It is formed by your immune system
- Hydrogen peroxide (H2O2):Not a free radical itself, but easily converts to free radicals like OH, which then do the damage. Hydrogen peroxide is neutralized by peroxidase (an enzymatic antioxidant).
- Reactive Nitrogen Species (RNS) (NO):Nitric acid is the most important RNS.
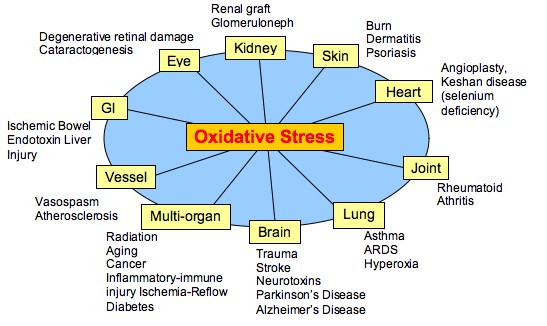
These various free radical species can damage DNA in different ways. They can disrupt duplication of DNA, interfere with DNA maintenance, break open the molecule or alter the structure by reacting with the DNA bases. Cancer, atherosclerosis, Parkinson's, Alzheimer's disease, and cataracts are examples of diseases thought to result from free radical damage. In fact, free radicals are implicated in more than 60 different diseases (see above) .
.
6. Lipid Peroxyl Radical. This radical unleashes a chain reaction of chemical events that can so totally compromise the cellular membrane that the cell bursts open, spews its contents, and dies. Lipids in cell membranes are quite prone to oxidative damage because free radicals tend to collect in cell membranes, known as "lipid peroxidation." When a cell membrane becomes oxidized by an ROS, it becomes brittle and leaky. Eventually, the cell falls apart and dies.
To conclude: a free radical is simply a molecule that is missing an electron. As soon as a molecule loses one of its attached electrons, it becomes unstable and seeks to re-stabilize itself by stealing an electron from the nearest molecule. This causes the attacked molecule to then become a free radical, and starts a chain reaction. Ultimately, as the process continues this can lead to cell damage.
Free radicals become extremely damaging to the body as they steal electrons from your cells. They begin to spread like a wildfire through the years if your body does not have enough antioxidants to keep them in check.
This is where antioxidants come in.
You may also like:
Understanding Antioxidants. How Do They Work?
Classification Of The AntiOxidants
The Most Common Sources Of Antioxidants
How Can We Recognize Antioxidant Rich Food?
How You Can Rate The Best Antioxidant? Is ORAC Score A Useful Tool?
How Antioxidants Affect Our Health In General